FAQs for Ethical Clearance
Home » Faculties of Science » Faculty Office »- Ethical clearance is required for any research project, or teaching activity that will form part of a research project (e.g.SOTL publications), that involve human participants, human tissues/samples, personal/private data, any animals (vertebrates or invertebrates), or animal tissues/samples.
- In the Faculty of Science, ethical clearance is also required for any practical experiment, as part of a teaching and learning activity, where animals are involved, or, where students are required to donate urine, saliva or blood samples.
No, all research projects, whether on undergraduate, Honours, Master’s, Doctoral, post-Doctoral, or staff level, requires ethical clearance.
Yes, ethical clearance cannot be issued retrospectively.
For studies involving human participants, using questionnaires only, the following documents must be submitted:
- Application form H1
- A copy of the questionnaire/s
- A copy of the information/consent form
- The completed POPIA impact assessment form
- Any other relevant supporting documents
For studies involving human participants, requiring the participants to do anything other than, or anything in addition to, filling out a questionnaire, including laboratory studies using donated samples from human participants, the following documents must be submitted:
- Application form H2
- A copy of the questionnaire/s (if applicable)
- A copy of the information/consent form
- The completed POPIA impact assessment form
- Any other relevant supporting documents
For studies involving animals or animal tissues/samples, the following:
documents must be submitted:
- Application form A1
- A copy of the necessary permits/permission letters (if available prior to ethical clearance) and any other relevant supporting documents
- Application form H1
- Application form H2
- Application form A1
- POPIA impact assessment form
- Information/Consent template
- Link to procure the latest SANS guideline document
- SOP documents for the HREC and AREC
- Committee composition and Chairperson contact details
- Flow-diagram showing the steps of how to apply for ethical clearance
- HREC and AREC meeting dates
Note: Please also refer to the flow-diagram and SOP documents provided under Question 5
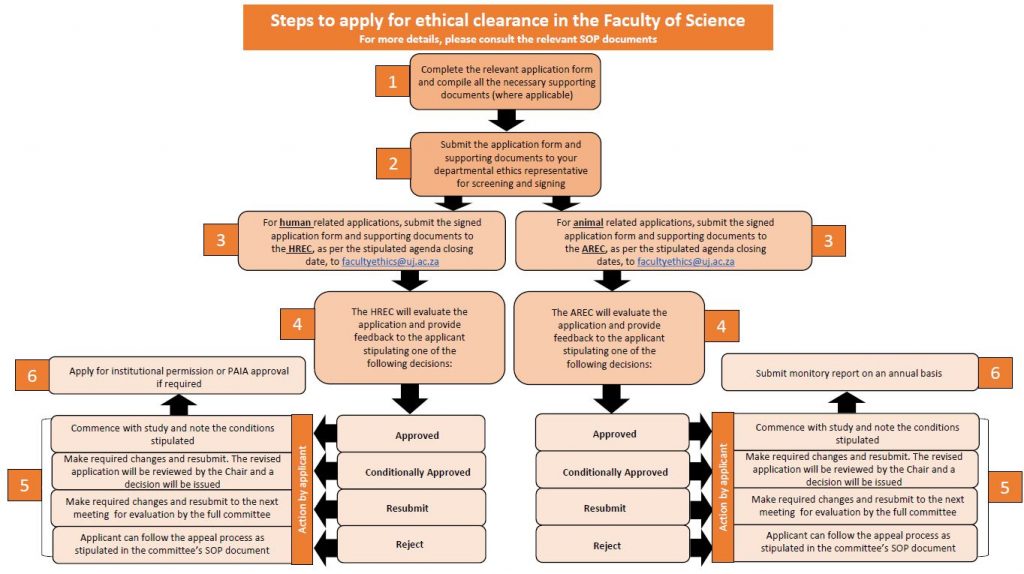
1. Application forms are available on the Faculty Website. Please always check the specific webpage for the latest version of the application forms (See Question 5).
2. The form/s must be completed in full by the applicant (Principal Investigator) and signed by all relevant parties, as applicable e.g.the student, the supervisor, collaborators etc.
3. The completed form and supporting documents (where applicable) must be submitted to the departmental ethics representative to screen and sign before submission to the committee.
4. If the study involve human participants, the completed, signed documents must then be submitted to the Human Research Ethics Committee (HREC) of the Faculty of Science. If the study involves animals, the completed, signed documents must then be submitted to the Animal Research Ethics Committee (AREC) of the Faculty of Science.
5. Both the HREC and AREC meets monthly, and the meeting dates and agenda closing dates are provided as part of the “Faculty Meeting Schedule” circulated by the Head of Faculty Administration. The dates are also provided under Question 5.
6. For more details on the process and the committee functioning, please refer to the respective Standard Operating Procedures (SOPs) of the respective committees (Available under Question 5).
7. Studies involving UJ students, staff or data, will require institutional permission following ethical clearance.
8. Studies requiring access to student records/data will require a PAIA application following ethical clearance.
All applications and supporting documents (HREC and AREC) must be submitted via email to:facultyethics@uj.ac.za. The contact details of the Chairperson/s can be found under Question 5.